Marketing Authorization Holder/ Designated Marketing Authorization Holder
Regulatory Affairs Service
To medical device and in vitro diagnostic product (IVD) manufacturers
who are planning to enter Japan market:
ICST supports both domestic and overseas manufacturers in entering Japan's medical devices and IVDs markets.
Regarding MAH and DMAH Service
The acquisition of Marketing Authorization Holder for medical devices and IVDs requires meeting numerous conditions, including personnel requirements, compliance with the QMS Ordinance, and the GVP Ordinance. We supports overseas manufacturers without a branch in Japan and both domestic and overseas manufacturers considering entering the medical device industry by acting as (Designated) Marketing Authorization Holder, providing support from regulatory application to post-market surveillance for medical devices and IVDs. We offer services as both MAH and DMAH.
MAH
Marketing Authorization Holder
The Marketing Authorization Holder (MAH) is responsible for consistently oversees the manufacturing, quality control, distribution, and post-market surveillance of medical devices and IVDs. They are responsible for ensuring compliance with regulations and assuring quality and safety throughout the entire process from manufacturing to market supply. When product approval is obtained, the MAH becomes the product license owner.
MAH is essential for distributing and selling medical devices within Japan.
DMAH
Designated Marketing Authorization Holder
The Designated Marketing Authorization Holder (DMAH) is responsible for consistently oversees the manufacturing, quality control, distribution, and post-market surveillance of medical devices and IVDs, but their scope of responsibility differs from that of the MAH. Unlike MAH, the product license owner in this case is the Foreign Manufacturer (Foreign Restrictive Authorization Holder).
When using the foreign restrictive approval system, a DMAH is essential.
ICST’s Regulatory Affairs Service
Below are some of the services we provide as MAH/DMAH.
Product application and notification
Foreign Manufacturer Registration (FMR)
Consultations with PMDA and Notified Bodies
General name etc. eligibility check
Surveillance audit correspondence
QMS (periodic) compliance application
Customs clearance correspondence
Issue instructions of shipment to distributors
Post-market surveillance work
Recall procedures for malfunctions and other issues
Medical insurance coverage support
Regulation check on advertisements etc.
based on PMD Act
Product Application Flow
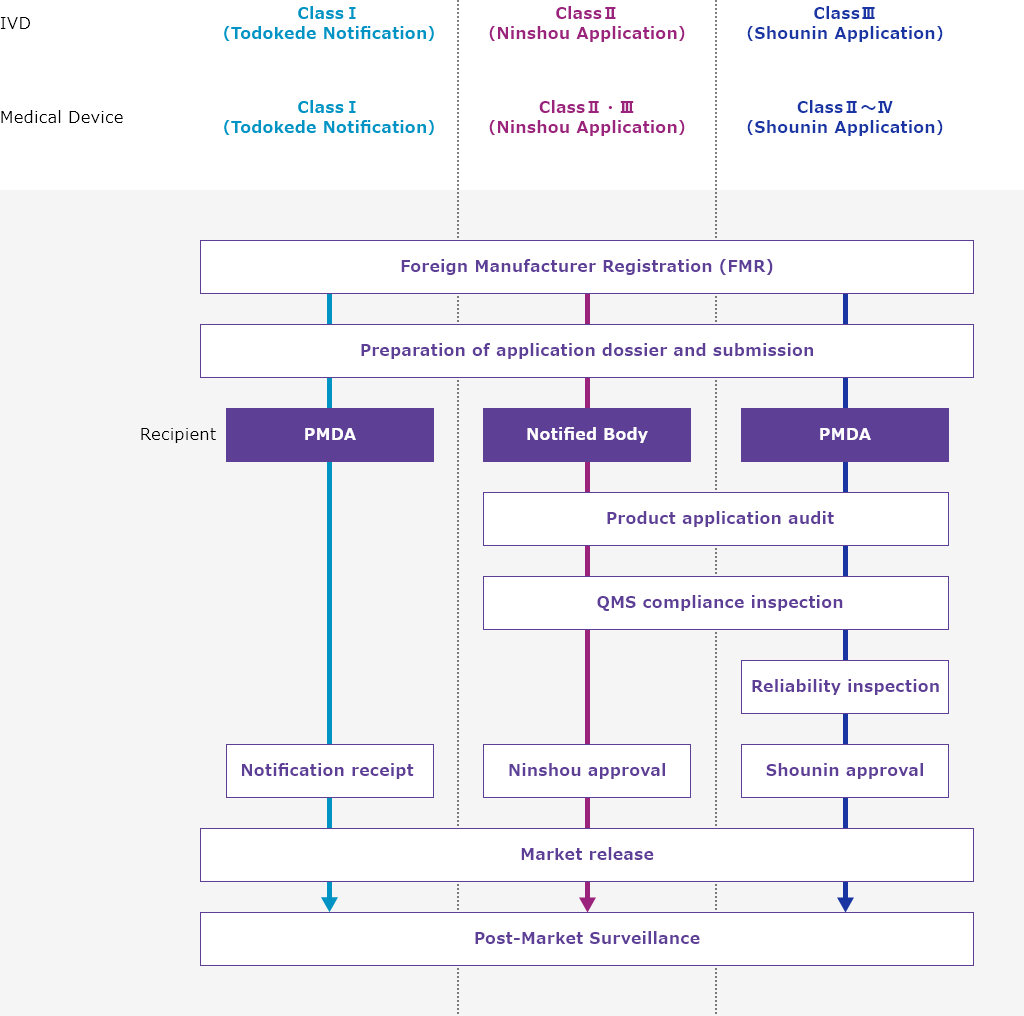
Features of ICST
One-Stop Service
By utilizing our Marketing Authorization Holder license and Manufacturing license, we handle the entire process from product application until approval to product inspection, packaging & labeling, and finally release product to the market.
English Communication
Our team has many English-fluent staff members, enabling us to communicate with you via emails and web meetings smoothly.
Regulation check on advertisements etc. based on PMD Act
We ensure that the materials for advertisement for the approved products comply with regulatory requirements in Japan.
Rich Experience
We have a wealth of experience in supporting the application of various medical devices. Our team of experts ensures swift and accurate applications.

D/MAH Key Responsibilities & Application Methods
You need to assign a local entity that has a MAH License to be able to register medical devices in Japan and import them. The local entity can either be your MAH or DMAH.
-
Marketing Authorization Holder (MAH)
- Key duties:
- ・Handle the FMR & product registrations and maintain the approvals
- ・Manage product quality & safety and ensure all of the manufacturing sites are compliant with Japan’s QMS Ordinance
- ・Manage post-market surveillance compliant with Japan’s GVP Ordinance
- ・Review and approve product release to the market
- ・Owner of product license
Etc.
- Marketing Authorization
- Domestic Quality Control Support
-
-
Foreign Restrictive Authorization Holder (FRAH)
- Key duties:
- ・Designate a MAH company
- ・Manage product quality & safety and ensure all of the manufacturing sites are compliant with Japan’s QMS Ordinance
- ・Owner of product license
- Marketing Authorization
- Quality Control

-
Designated Marketing Authorization Holder (DMAH)
- Key duties:
- ・Handle the FMR & product registrations and maintain the approvals
- ・Manage quality management specified for DMAH in Japan’s QMS Ordinance
- ・Manage post-market surveillance specified for DMAH in Japan’s GVP Ordinance
- ・Import products and manage domestic process with instructions from FRAH
- ・Review and approve product release to the market
- Domestic Quality Control Support
-
QMS : Quality Management System
GVP : Good Vigilance Practice
| MAH Application | DMAH Application | ||
| Marketing Authorization Holder | Designated Marketing Authorization Holder | Foreign Manufacturer | |
| Target Medical Device Class | Ⅰ~Ⅳ | Ⅱ~Ⅳ | |
| Target IVD Class | Ⅰ~Ⅲ | Ⅱ~Ⅲ | |
| Owner of product license | 〇 | × | 〇 |
| QMS Ordinance (Ordinance No. 169) | 〇 | △* | 〇 |
| GVP Ordinance (Ordinance No. 135) | 〇 | 〇 | × |
| Manufacturing process control | 〇 | × | 〇 |
| Control of Market Release | 〇 | 〇 | × |
*As stipulated in Clause 72 item 3 in QMS Ordinance No.169.
〇:Main responsibility
△:Limited responsibility
×:No responsibility
Outline of MAH Flow
- ・Target medical device:Class Ⅰ~Ⅳ
- ・Target IVD:Class Ⅰ~Ⅲ
- ・Owner of product license:ICST
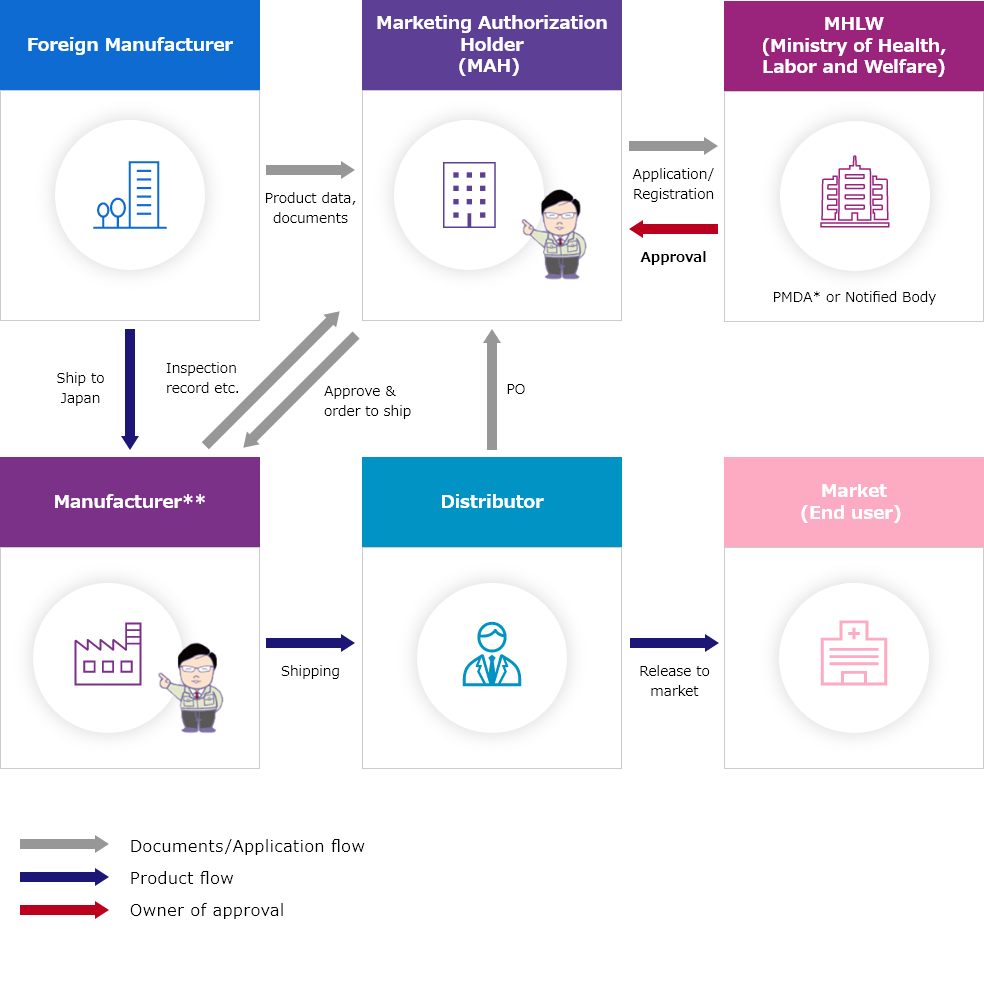
*PMDA:Pharmaceutical and Medical Devices Agency
**ICST's facilities or other domestic facilities. Can be discussed.
Outline of DMAH Flow
- ・Target medical device:Class Ⅱ~Ⅳ
- ・Target IVD:Class Ⅱ~Ⅲ
- ・Owner of product license:Foreign Manufacturer(FRAH)
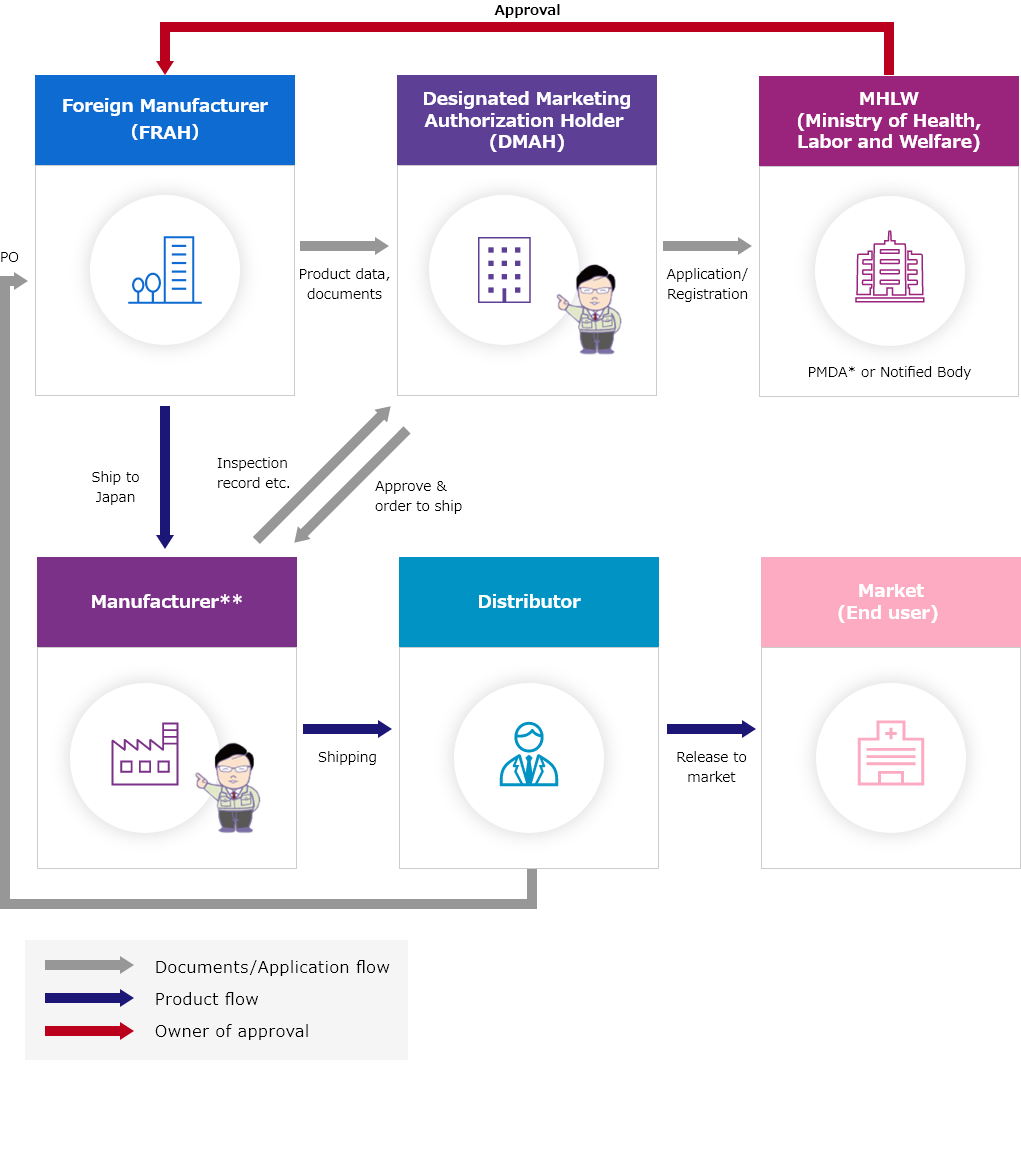
*PMDA:Pharmaceutical and Medical Devices Agency
**ICST's facilities or other domestic facilities. Can be discussed.
Rich Experience
Countries
- ・America
- ・Germany
- ・Belgium
- ・Canada
- ・Switzerland
- ・South Korea
- ・Sweden
- ・Italy
- ・China (Shenzhen, Dongguan, Shanghai)
- ・Denmark
- ・Netherlands
- ・Taiwan etc.
Products
- ・Insulin Subcutaneous Injection Syringe with Needle
- ・General-Purpose Imaging Diagnostic Device Workstation Program
- ・Home-Use Electrotherapy Device
- ・Multipurpose Ultrasonic Dental Device
- ・Genetic Analysis Device
- ・Pulsed Holmium YAG Laser
- ・Oxygen Concentrator
- ・Pulse Oximeter
- ・Low-Frequency Treatment Device
- ・Osmotic Pressure Analyzer etc.
Medical Devices/IVDs
ICST's Final Product Storage and Manufacturing Facility Service
(One-Stop Service)
What is final product storage facility?
Before releasing products to the market, MAH/DMAH must conduct a market release judgment. The facility where the products are stored during this release judgment process is called the "Final Product Storage Facility" (also referred as “Domestic Manufacturer”), and this facility must be registered as Manufacturing Facility.
We not only support product applications for medical devices and IVDs, but we also provide comprehensive support after product approval, including custom clearance support, storage, packaging & labeling, product inspection, and shipment to market. If needed, based on discussion, we can also provide support for sales and repairs for the products.
We provide this service in addition to our MAH/DMAH service.
Domestic
Manufacturer
Service
- ・Incoming inspection
- ・Inventory management
(Serial number, Expiration date, etc.) - ・Packaging & labeling
- ・Temperature-controlled storage
(Refrigerated storage available)
- ・Product (Quality) inspection
- ・Product receipt and shipment
- ・Confirmation of accompanying items etc.
Outline of One-Stop Service Flow
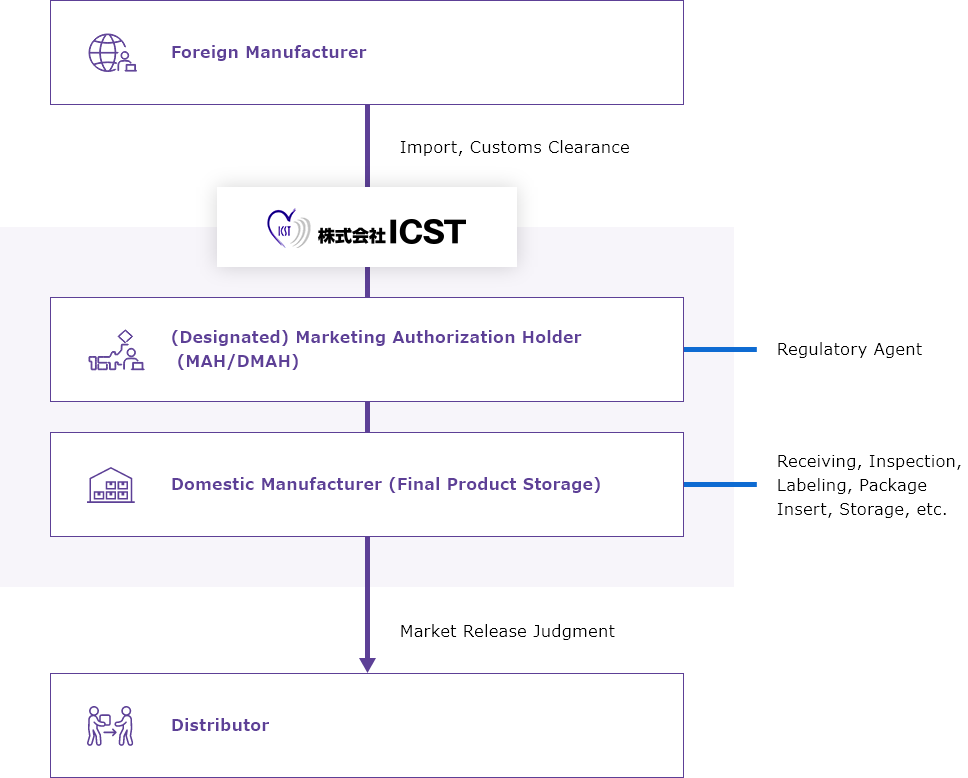
*Above is the model case when using both our MAH/DMAH and Domestic Manufacturer Services.
We can also accommodate your designated storage facility.
Pricing System
MAH/DMAH
In addition to the application support fees, we also charge a fixed monthly "Management Fee".
The contents of the Management fee are as follows:
- ・General marketing authorization holder operations
- ーQuality Management System (QMS) operations
- Management of product-related documents and records, etc.
- ーGood Vigilance Practice (GVP) operations
- Example: Collect and analyse products’ safety information, etc.
*Fee per product (discounts available as the number of products increases)
*The management fee is not affected by the products’ purchase quantity or total invoice amount.
The management fee is determined based on the product characteristics, risk analysis-based defect rate, and annual shipment volume etc..
Domestic Manufacturer
We charge the following for Domestic Manufacturer Service:
- ・Management fee
- ・Product inspection, packaging & labeling costs
- ーIncoming inspection
- ーConfirmation of accessories/documents
- ーAddition of Package Insert
- ーIssuance and attachment of Japanese rating plate,labels, etc.
- ・Storage fees (based on usage area and days)

